
- Chinese: Business Department 0539-8782638
- English: chau@dengzhuochem.com

- Chinese: Business Department 0539-8782638
- English: chau@dengzhuochem.com

Nickel-Alumina Catalysts for Hydrogen Production via Reforming
2025-12-03
In the quest for clean energy, hydrogen stands out as a promising carrier. However, producing it efficiently and sustainably from common resources is key. This is where catalysts, the unsung heroes of chemical reactions, play a pivotal role. Among them, nickel-based catalysts supported on alumina (Ni/Al₂O₃) are workhorses in industrial hydrogen production, particularly through reforming processes like steam methane reforming (SMR) and ethanol steam reforming (ESR).
Why Reforming? And Why Hydrogen?
Reforming is a chemical process that extracts hydrogen from hydrocarbon or oxygenated hydrocarbon feeds. It's a crucial step in bridging our current fossil-based economy and a future renewable one. Hydrogen produced can then be used in fuel cells for electricity, in ammonia production, or to upgrade biofuels.
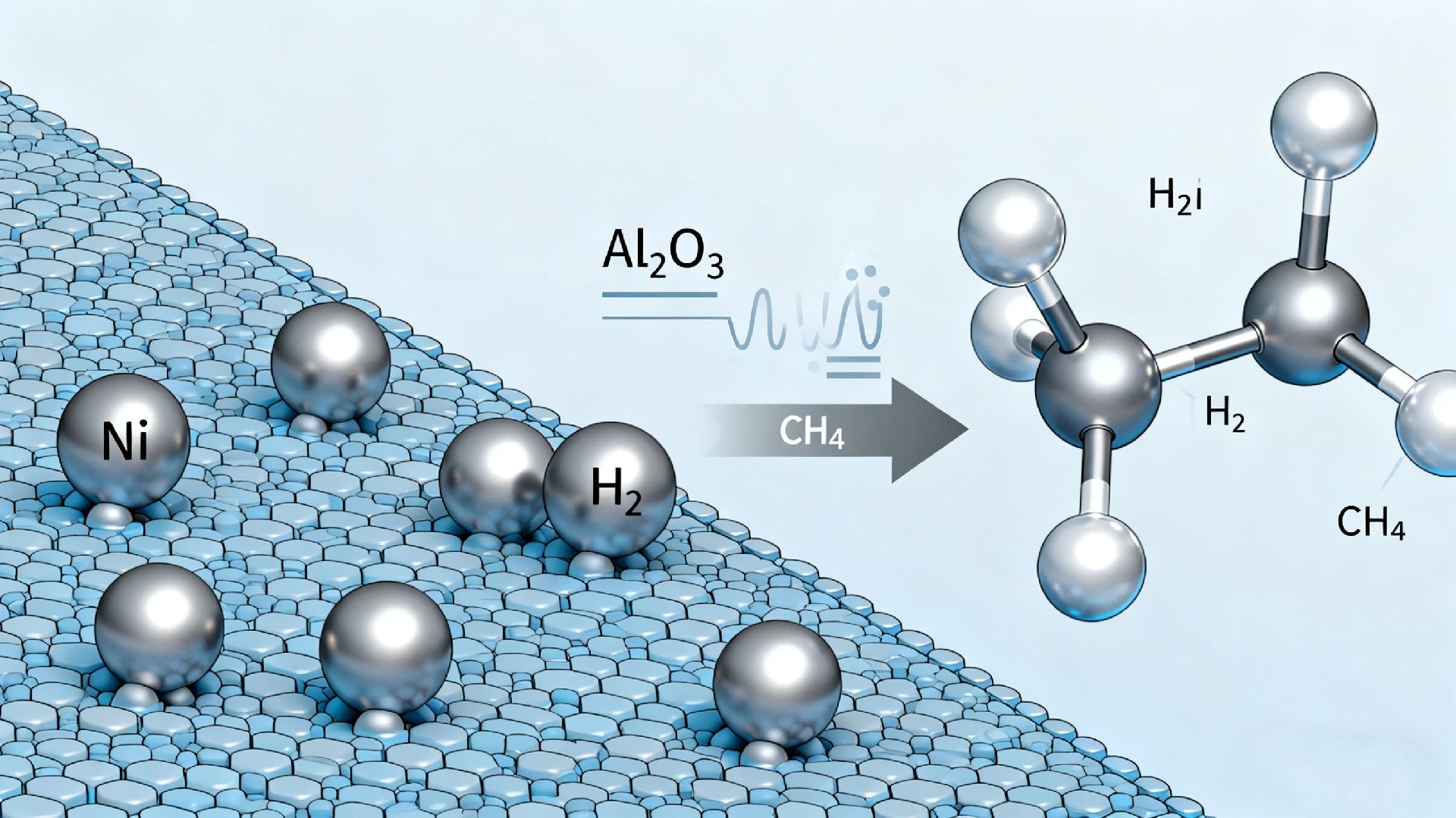
The Nickel-Alumina Catalyst: A Synergistic Partnership
At the heart of these reforming reactions lies a simple yet powerful combination:
Nickel (Ni): The active metal. Nickel is highly effective at breaking the C-H and C-C bonds in methane and ethanol. It's a cost-effective alternative to precious metals like platinum or palladium, offering excellent activity for hydrogen production.
Alumina (Al₂O₃): The support. This porous, high-surface-area material does more than just hold the nickel particles. It provides structural stability, prevents tiny nickel particles from sintering (clumping together) at high temperatures, and can even participate in the reaction mildly. Its acidity also helps in cracking larger molecules.
1. Steam Methane Reforming (SMR): The Industrial Giant
SMR is the dominant method for global hydrogen production, primarily using natural gas (methane, CH₄) as feed.
The Reaction: CH₄ + H₂O → CO + 3H₂ (This is followed by a "water-gas shift" reaction to produce more H₂).
Catalyst's Role: Ni/Al₂O₃ catalysts facilitate this reaction at high temperatures (700-1000°C). The alumina support ensures the nickel remains dispersed and stable under these harsh conditions. The main challenge here is carbon formation (coking), where carbon deposits can deactivate the catalyst. Modern Ni/Al₂O₃ catalysts are often doped with promoters (e.g., magnesium, cerium) to suppress coke and enhance durability.
2. Ethanol Steam Reforming (ESR): The Renewable Pathway
ESR offers a route to "green hydrogen" from bio-ethanol, a renewable liquid derived from biomass like sugarcane or corn.
The Reaction: C₂H₅OH + 3H₂O → 2CO₂ + 6H₂ (idealized).
Catalyst's Role: The process is more complex than SMR. Ethanol can decompose into various byproducts. Here, the acidic and structural properties of the alumina support become even more critical. They help in adsorbing and activating ethanol and water. The nickel sites then efficiently reform these intermediates into hydrogen. The challenge in ESR is not just coking but also managing selectivity to minimize unwanted byproducts like methane or acetaldehyde. Tailoring the alumina's properties and adding basic promoters are common strategies.
Key Advantages of Ni/Al₂O₃ Catalysts:
High Activity: Excellent at H-H and C-H bond activation.
Cost-Effective: Nickel is abundantly available.
Stable: Alumina provides thermal and mechanical robustness.
Adaptable: Their properties (nickel particle size, alumina pore structure, acidity) can be tuned for specific feeds like methane or ethanol.
Looking Ahead: Innovation and Sustainability
Research continues to improve these catalysts. Efforts focus on:
Enhancing resistance to coking and sintering.
Improving low-temperature activity for energy savings.
Utilizing biogenic or waste-derived feeds with ethanol reforming.
At their core, nickel-alumina catalysts exemplify elegant materials engineering, enabling efficient hydrogen production from both conventional and renewable sources. As the hydrogen economy evolves, so too will these versatile catalysts, continuing to underpin a critical link in our energy transition.
<< Previous Page
Next Page >>