
- Chinese: Business Department 0539-8782638
- English: chau@dengzhuochem.com

- Chinese: Business Department 0539-8782638
- English: chau@dengzhuochem.com

Desulfurization Made Simple: A Quick Guide to Hydrotreating and Non-Hydrotreating Catalysts
2025-09-01
In today’s refining and natural gas industries, cleaning up sulfur compounds from fuels is more important than ever. That’s where desulfurization catalysts—specifically Hydrotreating Catalysts (HDS) and Non-Hydrotreating Catalysts—play a vital role.
At a glance, these catalysts help remove sulfur, protecting the environment and ensuring better fuel quality. But how do they work, and which one is right for your operation?
What Are Desulfurization Catalysts?
Desulfurization catalysts are substances designed to remove sulfur-containing compounds—like mercaptans and hydrogen sulfide—from petroleum products or natural gas.
There are two main types:
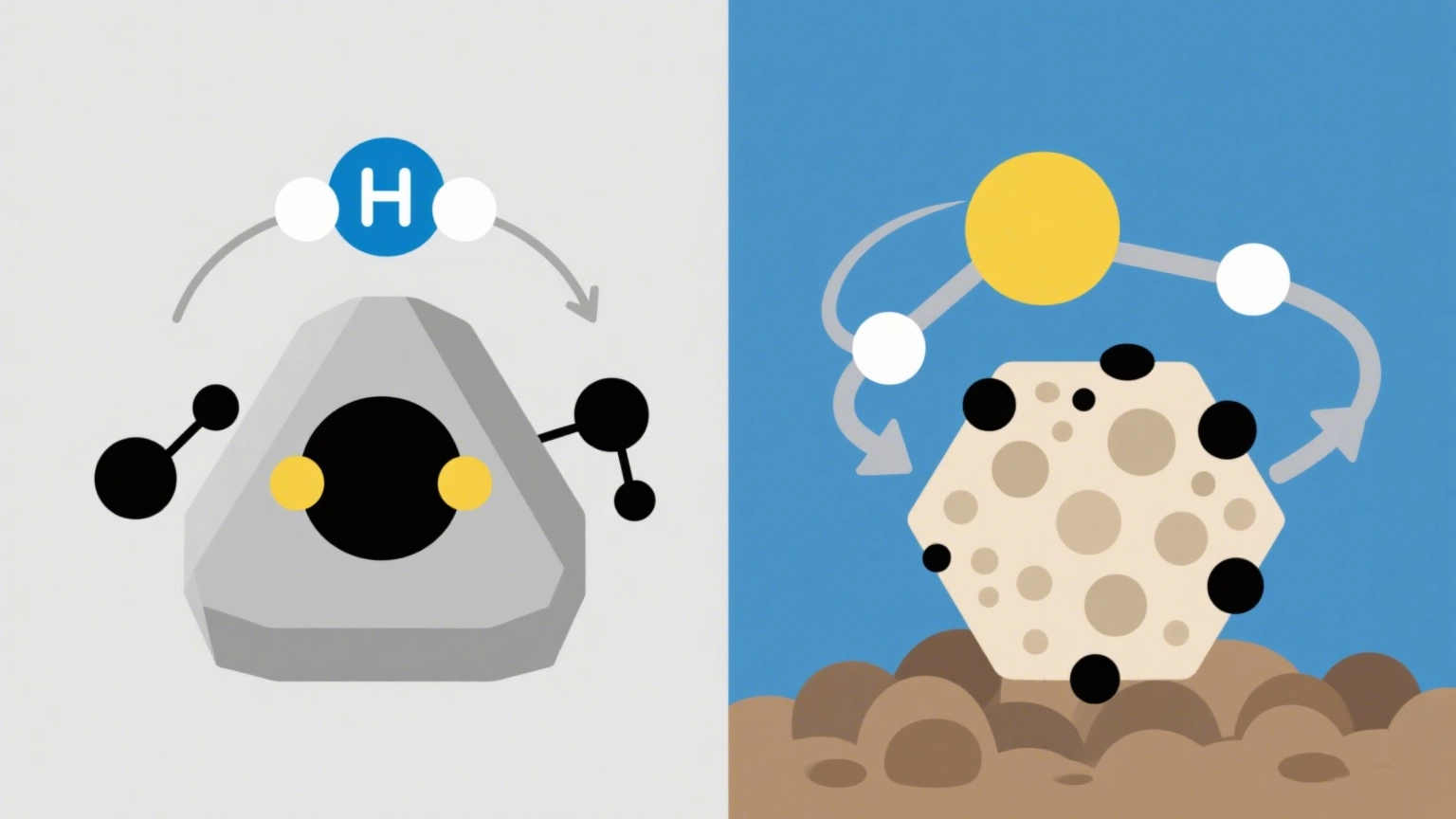
1. Hydrotreating Catalysts (HDS)
Hydrotreating uses hydrogen gas under high temperature and pressure to break sulfur-carbon bonds in fuel.
How it works: Hydrogen reacts with sulfur compounds to form hydrogen sulfide (H₂S), which is then separated.
Common applications: Refining of diesel, gasoline, and jet fuels.
Key advantage: Deep desulfurization; produces ultra-low-sulfur fuels.
2. Non-Hydrotreating Catalysts
These catalysts work without hydrogen, often through adsorption or chemical conversion.
How it works: They bind to sulfur compounds or convert them into harmless solid byproducts.
Common applications: Natural gas treatment, LPG, and niche refining processes.
Key advantage: Works in settings where hydrogen isn’t available or practical.
Which One Should You Choose?
Use Hydrotreating (HDS) if you’re refining petroleum products and need to meet strict low-sulfur standards.
Use Non-Hydrotreating catalysts for natural gas sweetening, biogas purification, or situations where hydrogen supply is limited.
<< Previous Page
Next Page >>